Introduction: Chimeric antigen receptor T-cell therapy (CAR T) is an effective therapy in relapsed/refractory (R/R) large B-cell lymphoma (LBCL) but is associated with prolonged cytopenias, persistently low CD4 T-cells, and hypogammaglobulinemia (defined as serum IgG <500 mg/dL) resulting in an immunocompromised state post CAR T [Logue 2021]. Prophylactic antimicrobials and intravenous immunoglobulin (IVIG) have been utilized to mitigate infectious complications, but practices are not standardized. We sought to examine patterns of immune reconstitution and their impact on incidence, timing, and management of infection following CAR T.
Methods: We identified patients (pts) with R/R LBCL treated with CAR T between 2015 - 2022 across 13 academic institutions. Data on measures of immune recovery and infections were captured post CAR T. Time-to-event curves were established by Kaplan-Meier method.
Results: 582 pts were included with a median age of 62 yrs (range 19-89). Pts were heavily pretreated with 3 median lines (range 1-18), and 26% with prior autologous transplant. Median time to ANC > 500 cells/mm3 was 11 days (D); median CD4 counts were 155 and 223 cells/mm 3 at 6 and 12 mo, respectively. Median absolute neutrophil and lymphocyte counts at 6 mo were 2300 and 690 cells/mm 3, respectively. At D90 post-CAR T, 61.5% (136/221) of pts had hypogammaglobulinemia (HGG) that persisted in 62.1% (90/145) at 1yr and 48.3% (56/116) at 2yrs.
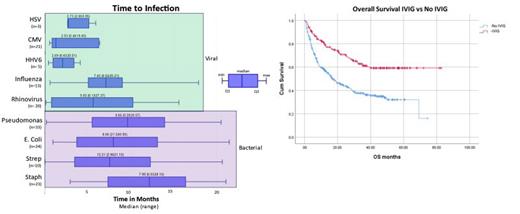
Data for D90-2 yrs post CAR T were analyzed to characterize late effects of CAR T as follows: Figure 1 describes time to first infection by organism of interest. 66.8% (193/389) of pts developed bacterial infections; median time to infection was 6.7 mo. Most common organisms were Pseudomonas (n =33), E. Coli (n=24), S. Aureus (n=22), C. Diff (n=26), Klebsiella (n= 18). HGG at D30 and D90 was associated with increased risk of bacterial infection, p = 0.002 (OR 2.5) and p= 0.013 (OR 2.1), respectively. Low CD4 counts and neutropenia at any time point within 2 years, did not correlate with increased rate of bacterial infection.
Excluding COVID, 31.2% (138/443) of pts developed viral infections; median time to infection was 5.4 mo. Most common organisms were COVID (n=49), Rhinovirus (n= 20), CMV (n= 21), and Influenza (n= 13). Median time to COVID infection was 15.6 mo (range 0.2- 33.1). Neither neutropenia, nor low CD4 counts within 2 yrs post CAR T correlated with increased rate of viral infection. HGG at any time point within 6 mo post CAR T was associated with increased risk of viral infections, including COVID (p = 0.04, OR 2.2). Fungal and PJP infections were infrequent, occurring in 19 and 6 pts, respectively. Median time to fungal infection was 1.7 mo.
For infection risk mitigation: 98.4% (496/504) of pts received prophylactic antivirals for median duration of 9.0 mo (0.2- 80.0) and 74.5% (371/498) received PJP prophylaxis for median duration of 6.3 mo (0.2-62.6). IVIG was administered in 34.3% (192/560) of pts with median time to initiation of 3.9 mo post CAR T. Pts with infection D90- 2 yrs were more likely to receive IVIG than those without infection (43.5% vs 26.9% respectively, p= 0.001).
Median follow-up in living pts was 35.3 mo with median OS of 27.8 mo (CI 21.2-34.5). Ninety-six (16.5%) pts received salvage therapy post CAR T. Infection of all types <90 days post- CAR T was associated with inferior OS (16 mo vs 43.3 mo, p=0.001). Infection >90 days-2 yrs post CAR T was not associated with worse OS. Presence of HGG at any time point did not impact OS (p = 0.218). However, median OS was not reached in pts who received IVIG vs 16.9 mo in pts who did not (Figure 2, p= 0.001). Furthermore, in pts with HGG, lack of IVIG utilization at D90 was associated with worse OS (p <0.001).
Conclusions: Immune suppression is a long-term toxicity of CAR T. Bacterial infections were common and most occurred within ~ 7 mo of infusion while viral infections were less frequent and occurred earlier. Both were associated with prolonged HGG. These observations should inform use of early antimicrobial prophylaxis. The majority of pts received anti-viral and PJP prophylaxis which may have contributed to lower rates of these infections. Pts with infection were more likely to receive IVIG with administration of IVIG improving survival. Our results support continued use of antiviral and PJP prophylaxis and the consideration of empiric IVIG within 30 days following CAR T to mitigate risk of infection and improve survival following CAR T.
Disclosures
Epperla:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding, Speakers Bureau; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Shouse:Kite Pharmaceuticals: Consultancy, Speakers Bureau; Beigene, Inc.: Speakers Bureau. Romancik:Astra Zeneca: Consultancy; KITE: Consultancy. Moyo:Kite Pharmaceuticals: Consultancy. Kenkre:Epizyme: Research Funding. Ollila:ADC Therapeutics: Honoraria; Ono Pharmaceuticals: Honoraria, Research Funding. Hess:Bristol Myers Squibb: Consultancy; ADC Therapeutics: Consultancy. Bhansali:Alva10: Consultancy. Ma:Janssen Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding; Genentech: Consultancy; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Juno/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Eli Lilly and Company/Loxo Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Winter:Merck & Co., Inc., Rahway, NJ, USA: Research Funding. Stephens:AbbVie: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Lilly: Consultancy; Novartis: Research Funding. Danilov:Janssen: Consultancy; Bristol Meyers Squibb: Consultancy, Research Funding; GenMab: Consultancy, Research Funding; Genentech: Consultancy; Astra Zeneca: Consultancy, Research Funding; Merck: Consultancy; Cyclacel: Research Funding; Beigene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Bayer: Research Funding; Nurix: Consultancy, Research Funding; Lilly Oncology: Consultancy, Research Funding; MEI: Consultancy, Research Funding. Shah:Umoja: Consultancy; Janssen: Consultancy; Tundra Therapeutics: Current holder of stock options in a privately-held company; LOXO-Lilly: Consultancy, Other: Travel support; Novartis: Consultancy; BMS/Juno: Consultancy; TG therapeutic: Consultancy; Epizyme: Consultancy; Seattle Genetics: Consultancy; Gilead/Kite: Consultancy; Incyte: Consultancy; Abbvie: Consultancy; Lilly Oncology: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Other: Travel support, Research Funding. Barta:Affimed: Consultancy; Acrotech: Consultancy; Daiichi Sankyo: Consultancy; Janssen: Consultancy. Torka:Genentech: Consultancy; Genmab: Consultancy; ADC Therapeutics: Consultancy; TG Therapeutics: Consultancy; Seagen: Consultancy; Lilly USA: Consultancy. Cohen:BMS/Celgene: Research Funding; Novartis: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Gordon:Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceuticals: Consultancy; Zylem Biosciences: Other: co-founder; Janssen: Other: data and safety monitoring board ; nanoparticles: Patents & Royalties: nanoparticles for cancer therapy (HDL NP As Inducers of Ferroptosis in Cancer, PCT/US2020/051549; Nanostructures: Patents & Royalties: Nanostructures for Treating Cancer and Other Conditions, PCT/US2013/027431). Karmali:AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Miltenyi: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Calithera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech/Roche: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Morphosys: Consultancy, Speakers Bureau; Janssen: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal